Our Business
iCM Project
We are developing a definitive therapy for severe heart failure patients with limited therapeutic options.

What is iCM
iCM is an iPS cell-derived cardiomyocyte created by a T-CiRA's project led by Dr. Yoshinori Yoshida, Associate Professor at CiRA, Kyoto University. The myocardial differentiation method, which has been studied at CiRA, has been further improved toward practical use by utilizing the cardiomyocytes purification method by small molecule compound discovered in the T-CiRA program. By transplanting iCMs, considered to be highly engraftable, pure, and safe cardiomyocytes, into the patient's heart, they are expected to replenish the cardiomyocytes lost as the disease progresses and promote remuscularization. Restoring the cardiac function of patients with severe heart failure, which has been considered difficult to treat, can be expected to improve the quality of life (QOL) and prognosis of these patients.
MD, PhD
Scientific Adviser
Associate Professor,
Center for iPS Cell Research
and Application (CiRA),
Kyoto University

Solution with iCM
-
Achievement of both high productivity and high purity.
Mass production-friendly suspension culture system. Removal of non-cardiomyocytes that could induce adverse effects, such as arrhythmia and cancer risk. -
Stable supply to the people who need it when they need it.
Cryopreservable and off-the-shelf cell product -
Assisting cardiac function for a long period of time.
High efficacy can be achieved with our highly efficient engraftable cardiomyocytes beyond the current drug therapy.
Cardiomyocyte therapy requires the delivery of hundreds of millions of cardiomyocytes or more to patients.
To provide cell products to patients with severe heart failure who are awaiting treatment, it is necessary to develop cell manufacturing technologies that can efficiently produce large numbers of cells. One of the most important technologies is a culture technique in which cells are suspended in a medium and cultured. Since the early stage of development, we have been working on a method for cardiomyocyte differentiation using a suspension culture system, which has been considered difficult to obtain high-quality cardiomyocytes. In addition, we have discovered unique small molecule compounds that efficiently remove non-cardiomyocytes generated during cardiac differentiation. By combining these state-of-art technologies with the differentiation method for preparing highly engraftable cardiomyocytes found in CiRA, high-performance, safe iCMs can be produced with a simple differentiation process. iCMs are highly effective when administered as single cells, eliminating the need for sheet or aggregate reformation. In addition, iCM is suitable for a wide range of administration methods, including catheter administration, enabling minimally invasive treatment with less burden on the patient.

iPIC Project
We are developing a definitive therapy with iPS cell-derived pancreatic islet cells (iPICs) to brittle Type 1 diabetes patients as a replacement therapy for islet transplantation.

What is iPIC
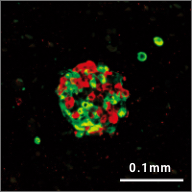
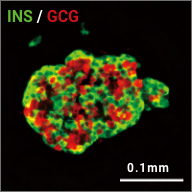
iPICs are human iPS cell-derived pancreatic islet cells expected to be suitable for cell therapy. They were discovered after a five-year optimization study through T-CiRA based on the pancreatic cell differentiation method (Stem Cell Res. 2015;14:185-97) discovered by Dr. Taro Toyoda, Junior Associate Professor at CiRA. iPIC is an aggregate of high-purity pancreatic endocrine cells that contains both insulin and NKX6.1 positive cells, a characteristic of pancreatic β cells. After implantation in vivo, pancreatic islet-like structures containing glucagon positive cells, another important endocrine cell type, are formed, and physiological insulin-secreting capabilities can be demonstrated in response to glucose loading and hypoglycemia.
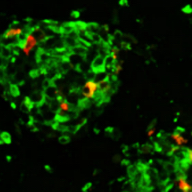

Maturation
in vivo
Center for iPS Cell Research
and Application (CiRA),
Kyoto University

Development of novel cell therapy product utilizing iPIC
To provide the novel treatment option to brittle Type 1 diabetes patients, we have established the original subcutaneous implantation method that supports the long-term engraftment of iPIC. So far, we have demonstrated proof-of-concept that implanted iPIC can treat diabetic rodents and pig models. High purity of expected endocrine cells, achieved by the combinatory approach of single-cell RNA sequencing-based transcriptomics and original compound-based purification, is one strength of our product. Fruitful collaboration with expertized equipment manufacturers enables us to yield billions of cells from one batch with a cell culture system optimized for iPIC. Now we are developing a product that can be supplied with the freeze-thaw tolerable iPIC for immediate use.
Solution with iPIC

islet-like cells (iPICs)

- Scalable culture process
- Homogeneous and stable quality
- High purity

- Pancreas islet-like morphology
- Physiological insulin secretion
- Less invasive and retrievable surgery

- Freedom from hypoglycemic events
- Independence of insulin injection
- Minimization of immunosuppress-ant use

We provide a wide range of services to support the development and research of regenerative medicine products involving iPS cells.
(For more details, please visit Platform Business Unit page.)
What is Platform Business?
Our Platform Business initiative represents a strategic fusion of two core strengths: our in-house expertise in the development of regenerative medicine products, and the advanced technical capabilities of specialists who have long supported cutting-edge iPS cell research. Through this integration, we have developed a suite of specialized services designed to meet the evolving needs of companies and research institutions working with iPS cells. Our mission is to support the sustainable growth and innovation of the global iPS cell community. With a team of seasoned professionals who possess extensive experience in handling iPS cells, we are uniquely positioned to provide tailored, high-value solutions that address each client’s specific challenges and goals. Partner with us to unlock new possibilities in iPS cell innovation.
Our Services
We offer three main types of services to meet your needs:
- Contract Research Services
- Comprehensive testing and research on iPS cell culture, differentiation, and analysis, performed on your behalf.
- Consulting Services
- In-depth investigations and analysis to solve technical challenges in iPS cell research and development.
- On-site Support Services
- Hands-on assistance from our skilled technical staff directly within your experimental environment.
For further details on each service, please visit our dedicated Platform Business Unit page.